Inside IB: Exploring Atomic Structure Through Inquiry

Inside IB: Exploring Atomic Structure Through Inquiry
An Inside IB Series Article.
In a hands-on, multi-station science activity, students were invited to investigate the question: “What makes one atom different from another?” Rather than passively receiving information, they became active participants in their learning journey. Through physical models and digital simulations, they explored atomic structure, discovering the roles of protons, neutrons, and electrons in shaping identity, mass, and charge.



Atoms, protons and neutrons are all quite common place in any high school classroom, however, we take commitment to the International Baccalaureate (IB) philosophy far beyond textbooks and assessments. This science lesson designed by Middle School Science Teachers Maria Hodorogea and Meisam Shahsavari and supported by Head of Department Hugo Pratt and Middle School Reuben Haggar exemplifies this beautifully, offering a compelling glimpse into the IB’s core principle of inquiry.
The IB principles provide a strong foundation for such innovative learning. They allow teachers to modify traditional structures so that students can take the lead in their own learning, gain ownership over their exploration of a subject, and expand their capacity for inquiry.
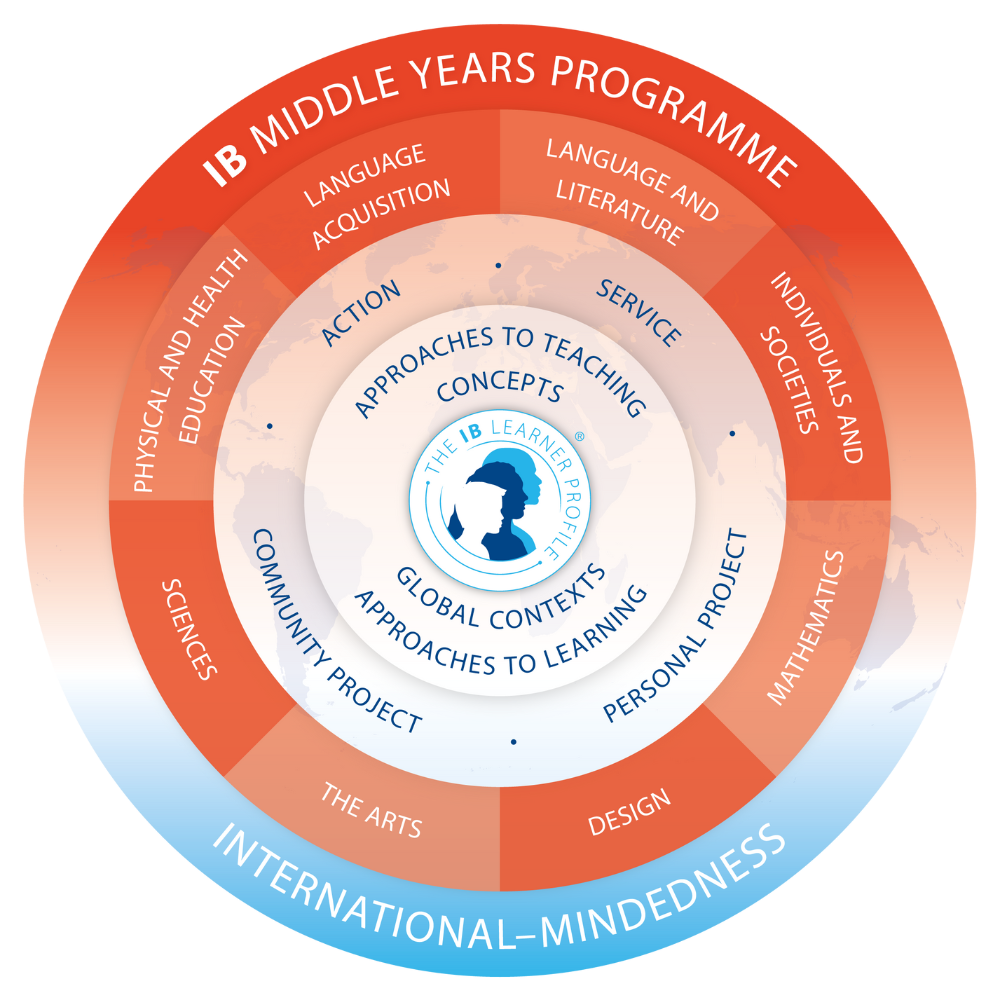
In IB programme, inquiry is more than a teaching method—it’s a guiding philosophy that shapes how students learn across all subjects. Rather than simply absorbing facts, students are encouraged to ask meaningful questions, explore ideas deeply, and construct their own understanding through investigation and reflection. Inquiry-based learning helps students connect prior knowledge to new concepts, develop critical thinking skills, and take ownership of their learning. It’s a dynamic process that fosters curiosity, creativity, and confidence—qualities we nurture every day at BWYA.
Students focused their inquiry through three interactive learning stations:
- Station 1: Isotopes – Creative Thinking in Action
Students compared Lithium-6 and Lithium-7 nuclei, then applied their observations to identify a mystery isotope. They weren’t given definitions, building understanding from evidence. - Station 2: Ion Formation – Inquiry at the Core
Using simulations, students manipulated atomic particles and observed real-time changes. They asked questions like, “What happens if I add one more electron?” and drew conclusions from their own data. - Station 3: Atomic Mass – Reflective Thinking and Real-World Application
Students calculated weighted averages and compared their results to the Periodic Table, deepening their understanding of how atomic mass is determined and why it matters.

“This wasn’t just about science content,” said Mr Shahsavari. “It was about developing the skills that make inquiry meaningful—creative thinking, research, reflection. Students weren’t just learning facts; they were learning how to learn.”
The activity also highlighted the IB’s Approaches to Learning (ATL) framework, which nurtures thinking, research, communication, and social skills. Students were encouraged to reflect on their findings, justify their reasoning, and record their learning in a way that promotes long-term understanding.
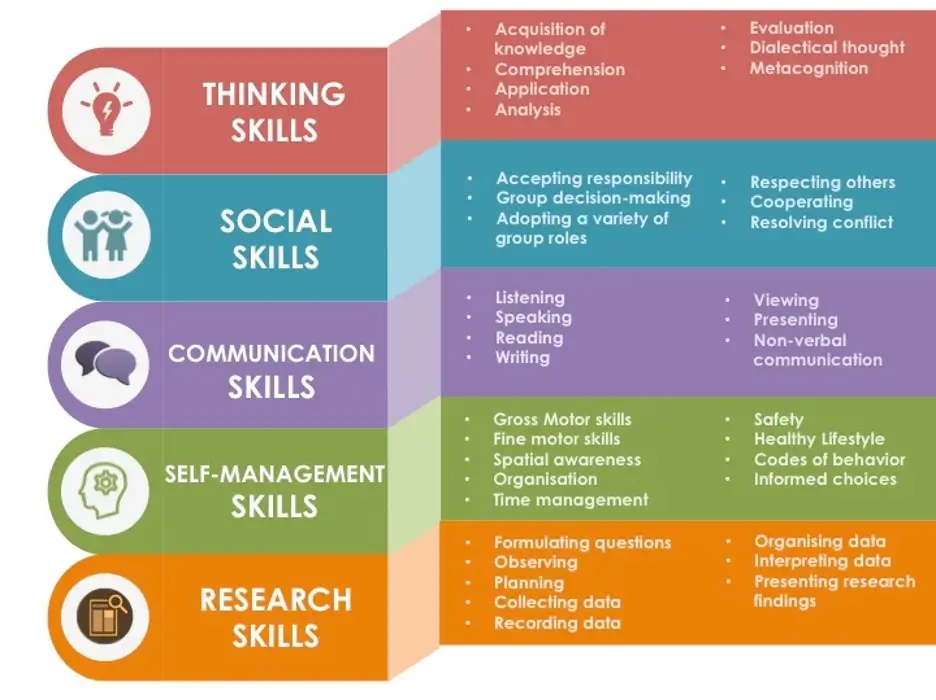
Mr Haggar added, “Inquiry is at the heart of the IB. It’s what transforms a classroom into a space of discovery. When students take ownership of their learning, they become more than students—they become thinkers, collaborators, and problem-solvers.”
This lesson is a powerful example of how BWYA brings the IB philosophy to life. It shows that inquiry is not just a method—it’s a mindset. It prepares students not only for academic success, but for thoughtful engagement with the world around them.

For parents considering an IB education at BWYA, this is the kind of learning environment your child will experience: one that values curiosity, encourages deep thinking, and fosters a lifelong love of learning.

